Abstract
Background SARS-CoV-2 infection has been associated with a poor prognosis in patients with Philadelphia-negative myeloproliferative neoplasms (MPN).(Passamonti et al. Lancet Haematol. 2020;7(10):e737-e745).
Nevertheless, given the concerns about SARS-CoV-2 vaccine-associated thrombosis (Pavord et al. N Engl J Med 2021;385(18):1680-1689) and the well-known thrombotic risk associated with MPN (De Stefano et al. Leukemia 2016;30(10):2032-2038), patients had frequently vaccine-hesitancy and some of them even refused to be vaccinated.
Methods To estimate the potential risk of thrombosis associated with SARS-CoV-2 vaccination in MPN, we evaluated 335 consecutive MPN patients vaccinated against SARS-CoV-2 with at least one dose.
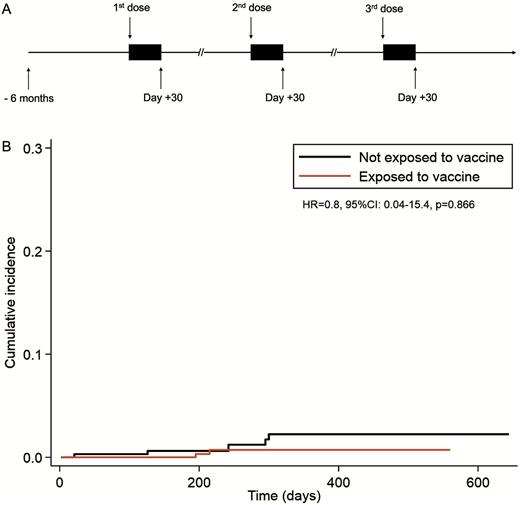
Proportional hazard Cox model for multiple failures was used to compare the risk of thrombotic complications according to vaccine exposure (treated as time-dependent covariate), and results of these models were reported in terms of Hazard Ratio (HR) for vaccine exposure vs non-exposure periods, together with its 95% Confidence Interval (95%CI). The exposure period was defined for each patient from the date of any SARS-CoV-2 vaccine dose till 30 days after; the non-exposure period included for each patient the six months before the first dose and the period beyond 30 days after each dose till last follow-up (see Figure 1A). To take into account time between diagnosis and enter date, left-truncation was applied.
Results Starting from January 2021 we enrolled 335 MPN patients, including 99 affected with PV, 176 affected with ET, 49 affected with primary or secondary myelofibrosis and 11 affected with MPN unclassifiable. Median age was 55 years (range 15-88); 153 (46%) were males and 182 (54%) were females. Considering the mutational status, 252 patients (75.2%) were JAK2 V617F-mutated, 55 patients (16.4%) were CALR-mutated, 6 patients (1.8%) were MPL-mutated and 22 patients (6.6%) were triple negative. Seventy out of 335 patients (20.9%) were not receiving cytoreduction at the time of vaccination; the remaining 265 (79.1%) were under cytoreductive treatment (226 with hydroxyurea, 22 with ruxolitinib, 13 with interferon, 4 with busulfan). A previous history of thrombosis was reported in 100/335 (0.3%). Almost all patients received at least two doses of vaccination against SARS-CoV-2 (331/335, 98.8%); 75% of patients (224 of 335) received also the third dose. In most patients (320/335, 95.5%) the first two doses consisted of mRNA-based vaccines; in only 15 out of 335 (4.5%) the first two doses consisted of adenovirus-vectored vaccines. In all cases the third vaccination was performed with mRNA-based vaccines. Thrombotic complications included only major thrombosis. The risk of thrombosis in the exposure period did not significantly differ from the risk of thrombosis in the non-exposure period (HR=0.8; 95%CI 0.04-15.4, P 0.866), as shown in Figure 1B. No case of cerebral venous thrombosis was reported in the post-vaccine period.
Conclusions Although these results need to be confirmed in larger studies, these data deliver an important message to the clinical community: SARS-CoV-2 vaccines do not increase the risk of thrombosis in MPN patients; thus, it is pivotal for health care professional to vaccinate the highest number of MPN patients, reassuring them regarding thrombotic complications.
Disclosures
Arcaini:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees. Rumi:Novartis: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.